During my master thesis period I worked on the genome assembly, annotation and phylogenomics of Midichloria mitochondrii, the first described intra-mitochondrial bacterium. M. mitochondrii is probably one of the most intriguing bacteria I ever met: it lives within the mitochondria of the oocytes of the hard tick Ixodes ricinus, occupying the space between the two membranes of the organelle. Surprisingly, the analysis of the M. mitochondrii genome led us to understand something important about the origin of mitochondria more than the evolution of the bacterium itself, but this is another story (see Sassera et al. 2011, https://doi.org/10.1093/molbev/msr159).
Years after I got the master degree, we had the opportunity to investigate one of the most interesting aspects of M. mitochondrii (in my opinion): its life cycle. Electron microscopy images clearly show mitochondrial cells colonized by one, two, three..up to about ten M. mitochondrii cells, and the highly “parasitized” ones having degraded structure. For this reason, it has been supposed that M. mitochondrii could behave similarly to a predatory bacterium. Following this hypothesis, the M. mitochondrii cells free in the oocyte cytoplasm invade the mitochondria and replicate within them until they lead to the organelle lysis (Sacchi et al. 2004, https://doi.org/10.1016/j.tice.2003.08.004).
To test this predatory-like hypothesis, we studied 71 I. ricinus oocytes from 11 ticks, counting the number of mitochondria parasitised by zero, one, two, three, … M. mitochondrii cells. Then, we used these values to model the bacterium life cycle within the oocyte. A total of 12,068 mitochondria and 7,805 “Ca. Midichloria mitochondrii” (intra-mitochondrial and free in the oocyte) units were observed.
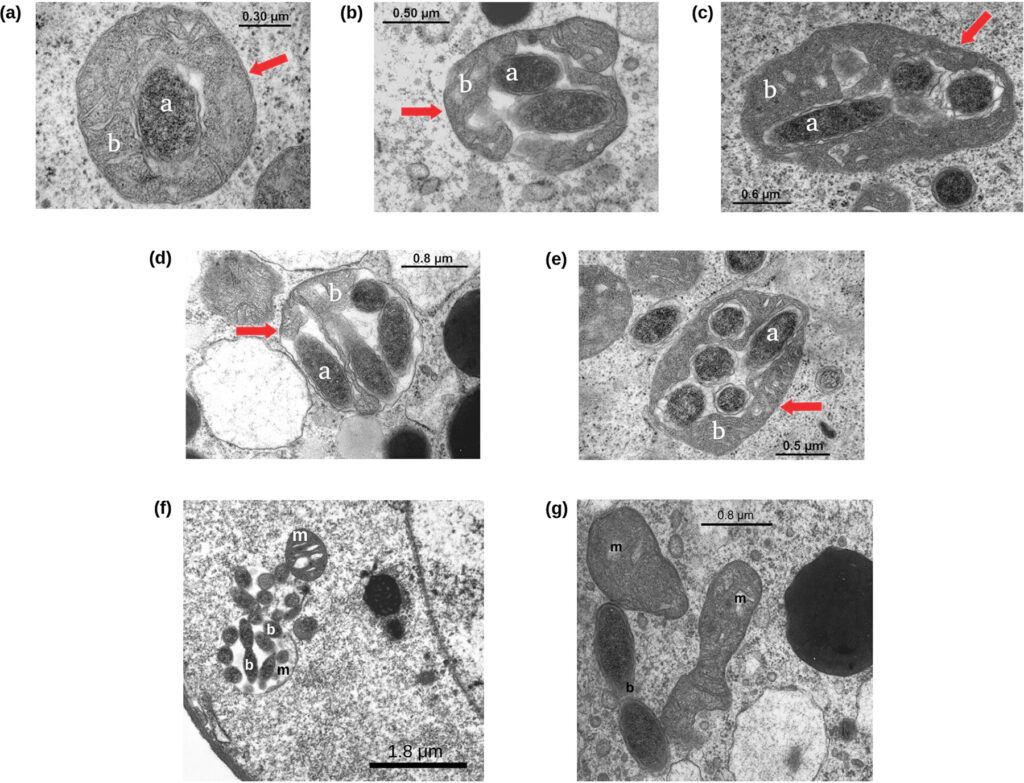
Surprisingly, the obtained results are not coherent with a predatory-like life cycle. We very rarely observed bacterial cells in replication, either within the mitochondria (two possible events observed) and in the cytoplasm (nine possible events). Furthermore, we observed a linear correlation between the frequency of cytoplasmic M. mitochondrii cells (normalized on the total number of oocyte M. mitochondrii) and the frequency of non colonized mitochondria (normalizes on the total number of mitochondria). In other words, we found that the more are the preys (i.e. non colonized mitochondria) the more are the predators (cytoplasmic M. mitochondrii). This peculiar linear relationship is quite unlikely in a pray-predators system, as we tested using the Lotka-Volterra model. Last but not least, it is very rare that a stable prey-predators system can emerge in a closed and limited space like an oocyte.
Thus, we try to change the point of view and we proposed another possible life cycle. Mitochondria have been found to be connected in a network in several organisms, in particular in humans. We hypothesized that the mitochondria of I. ricinus oocytes could be connected in a network and that M. mitochondrii could move, somehow, through it. Without experimental evidence we tested this hypothesis in silico using stochastic simulations. Our results show the the movement of
M. mitochondrii cells within an hypothetical mitochondrial network in coherent with the observed data: at the equilibrium, the number of mitochondria parasitized by zero, one, two, etc. bacteria are very similar to those observed by electron microscopy.
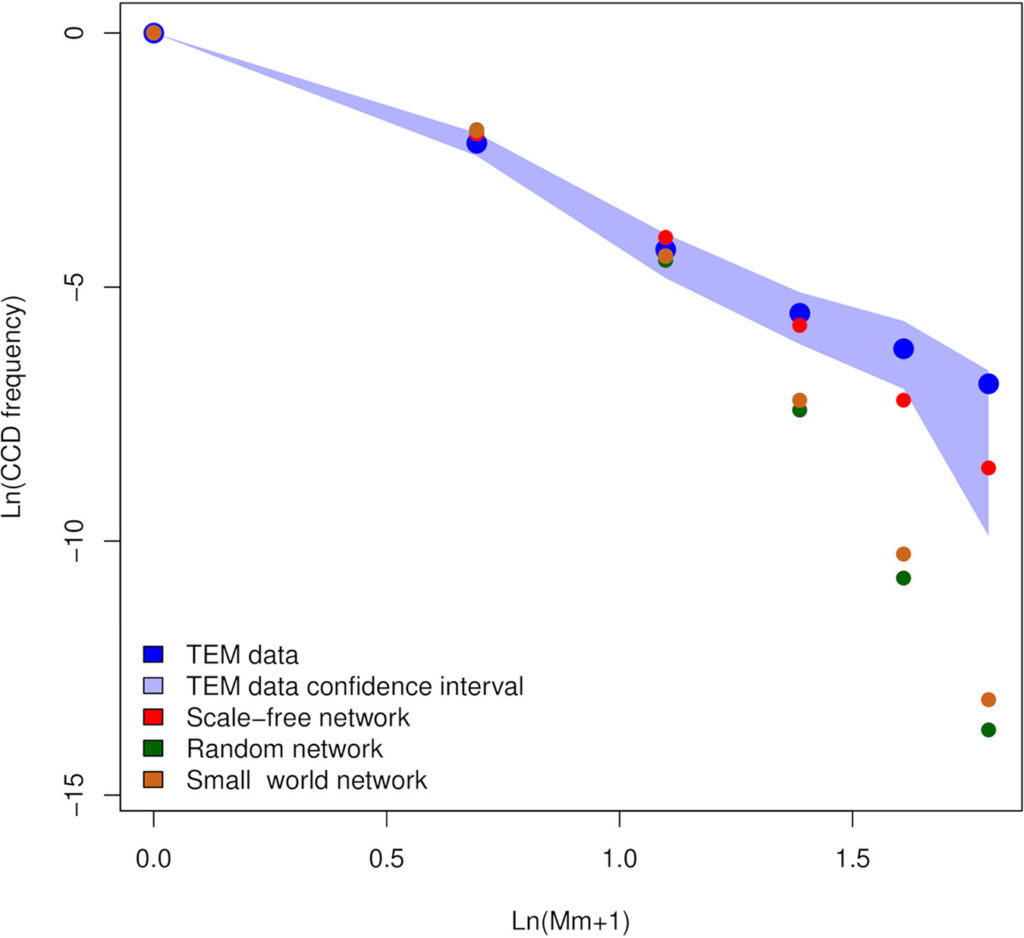
Can we say that M. mitochondrii moves through the oocyte mitochondrial network? At the state of the art, we can not be sure of this, also considering that our knowledge about I. ricinus mitochondrial network is very very limited. On the other hand, this work allowed us to understand that the predatory-like cloud is not the most fitting for
M. mitochondrii, while the bacterium could move within the I. ricinus mitochondrial network … even if this network really exists!
At the end, we scratched the surface of the problem and the life cycle remains an intriguing open question about this so interesting and difficult to study bacterium!